In Depth: Canceled Contracts Upset Chinese Drugmakers’ Overseas Push
Listen to the full version
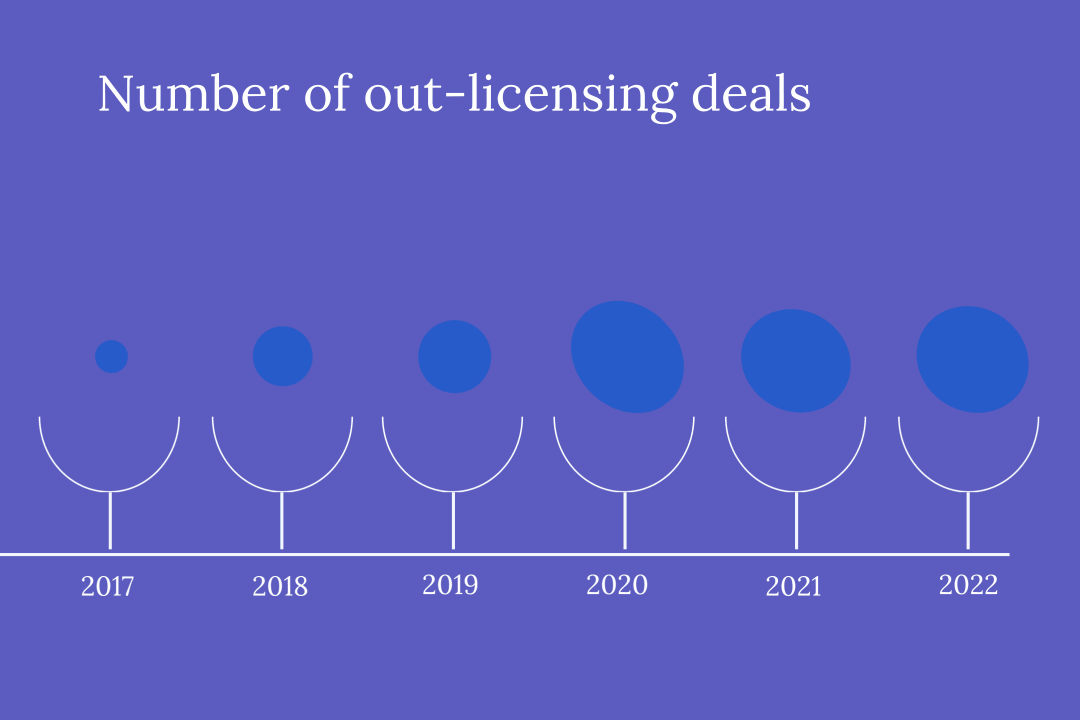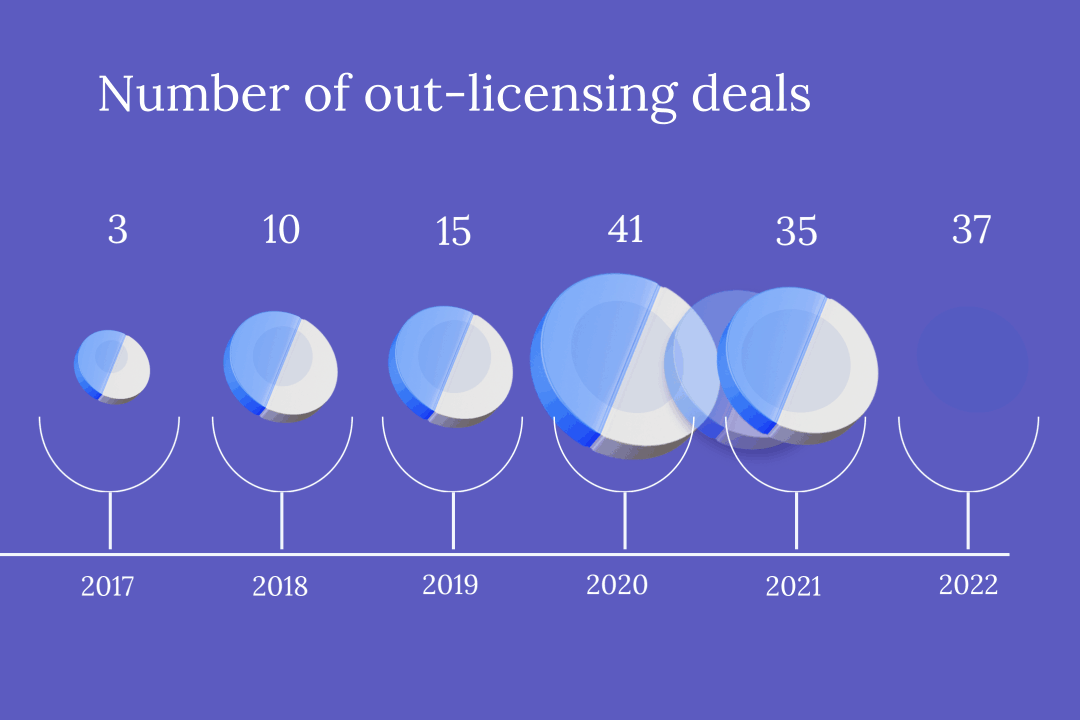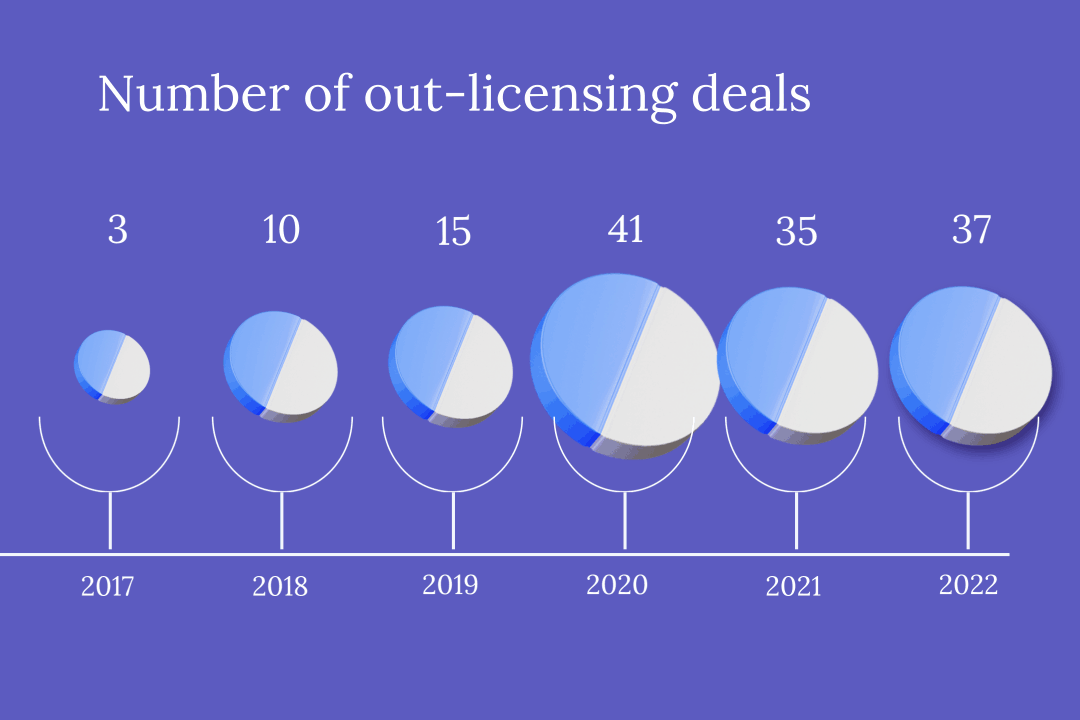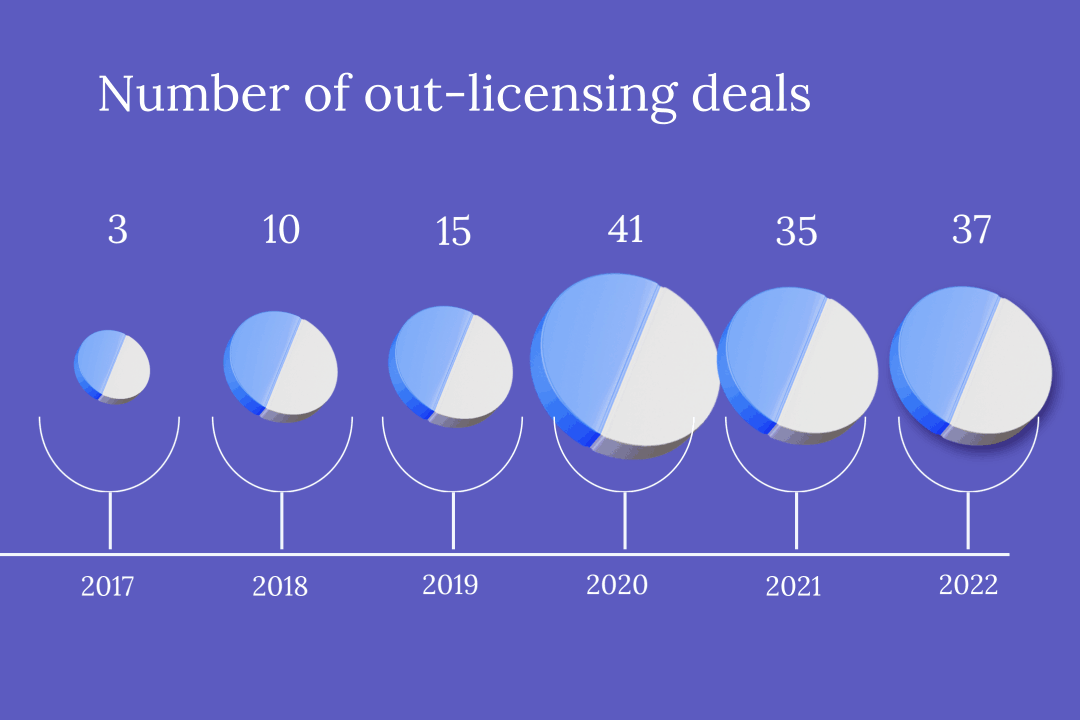
With ever tighter profit margins in the domestic market, China’s innovative drug developers are increasingly partnering with foreign companies to pitch their products overseas. But while this route of “out-licensing” drugs is lucrative, it can also be precarious, with multiple contracts terminated this year alone.
In the first half of this year, more than 20 Chinese pharmaceutical firms granted foreign partners the rights to commercialize novel drugs — including treatments already approved for use and still in development — in out-licensing deals worth a total of about $12 billion.
- PODCAST
- MOST POPULAR







 Sign in with Google
Sign in with Google
 Sign in with Facebook
Sign in with Facebook
 Sign in with 财新
Sign in with 财新