Do Patients Benefit As Domestic and International Weight Loss Drug Companies Battle it Out?(AI Translation)
Listen to the full version

文|财新周刊 陈曦 蒋模婷
By Caixin Weekly's Chen Xi and Jiang Moting
[Weekly Early Read] As Obesity Rises in China, Approaches to Weight Loss Evolve from Awareness to Treatment and Strategy
在内分泌科减重门诊用药九个月,32岁的简诚(化名)正在逐步停用“神药”司美格鲁肽。体重减轻12%后,反弹是他此刻最大的“敌人”。
After nine months of medication at the endocrinology weight loss clinic, 32-year-old Jian Cheng (alias) is gradually discontinuing the "miracle drug" semaglutide. Having lost 12% of his body weight, rebound is currently his biggest "enemy."
医生叮嘱他,发现反弹苗头要及时回去就诊,“别等又胖了几十斤才来看”。平时颇为关注减重药物消息的简诚,想好了“保底方案”——“如果后面再反弹,我还可以打替尔泊肽。”
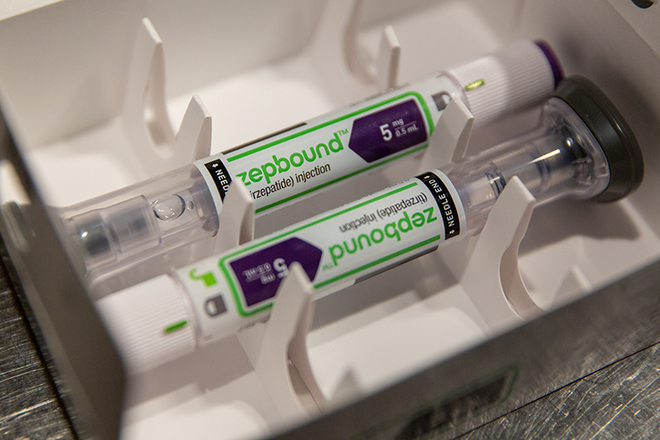
The doctor advised him to return for a consultation as soon as he noticed any signs of weight regain. "Don't wait until you've gained dozens of pounds again to come see me." Jian Cheng, who frequently keeps up with news about weight loss medications, has already planned a "fallback option"—"If I gain the weight back, I can always take tirzepatide."
- DIGEST HUB
- The rise of obesity in China has led to the adoption of modern weight loss medications like semaglutide and tirzepatide, approved for use in 2023-2024.
- Semaglutide ranked second in global pharmaceutical sales in 2023, with a $21 billion combined revenue, while tirzepatide, a strong competitor, saw rapidly growing sales.
- In China, a competitive landscape among Novo Nordisk, Eli Lilly, and domestic companies is emerging, driven by differentiated strategies in weight loss medication R&D.
The article delves into the evolving landscape of obesity treatment in China, emphasizing the shift from conventional approaches to modern pharmacotherapy. A central figure, 32-year-old Jian Cheng, symbolizes the modern patient navigating these new drug therapies, having successfully lost 12% of his body weight using semaglutide and planning a fallback with tirzepatide if weight regain occurs [para. 1][para. 2].
Semaglutide and tirzepatide, both from major pharmaceutical companies Novo Nordisk and Eli Lilly respectively, are the new focal points in weight-loss medication. Semaglutide started as a diabetes medication before gaining approval for weight loss [para. 3][para. 4]. Meanwhile, tirzepatide, a dual-target receptor agonist, gained approval for weight loss in China in July 2023 [para. 5][para. 6].
Historically, the development of obesity drugs has faced significant setbacks, with many initially approved medications being withdrawn due to safety concerns. Before the introduction of semaglutide and tirzepatide, Orlistat was the only weight-loss drug available domestically, despite its gastrointestinal side effects and limited effectiveness [para. 7]. The introduction of GLP-1 class drugs, which mimic bodily mechanisms regulating appetite and metabolism, has revolutionized obesity treatment, according to Ji Linong of Peking University [para. 8][para. 9].
Novo Nordisk and Eli Lilly lead the competitive landscape, with Novo Nordisk’s semaglutide achieving significant sales and discussion in 2023 despite being surpassed in total sales by a new "King of Drugs." The weight loss version of semaglutide alone boasted a 400% annual sales increase [para. 11][para. 12]. With the launch of tirzepatide, Eli Lilly quickly gained ground, signifying a fierce competition in China's populous obesity market [para. 14][para. 15].
For clinical experts, the differentiation in drug competition is becoming essential, with the focus shifting from just weight loss to overall health benefits like metabolic and cardiovascular improvements. This competitive fervor also raises questions about whether the weight loss market will genuinely aid obese populations or merely capitalize on market hype [para. 16][para. 17].
Novo Nordisk’s two GLP-1 receptor agonists, Liraglutide, and Semaglutide, highlight evolving strategies from glycemic control to weight management. Semaglutide's initial FDA approval was for glycemic control in 2017, transitioning to weight loss in 2021 under the brand Wegovy. In China, the weight management approval is expected in 2024 [para. 24][para. 25].
Experts indicate that although both Semaglutide and Tirzepatide effectively reduce weight, their clinical applications and the anticipated head-to-head clinical trial results will provide more definitive comparisons. This trial by Eli Lilly, comparing tirzepatide with semaglutide, aims to complete by December 2024 [para. 29][para. 30].
The article also reveals ongoing development in obesity drugs with companies like Pfizer, Boehringer Ingelheim, and Amgen competing by conducting their "head-to-head" trials against semaglutide [para. 47][para. 48]. Multi-target approaches, combining various action mechanisms, are also being explored to enhance efficacy and reduce side effects [para. 44][para. 45].
Domestically, numerous Chinese companies are pushing their GLP-1 drugs through various clinical trials, reflecting significant research and development momentum. The competitive environment includes a mix of innovative approaches and traditional generic drug development as patents for established drugs like semaglutide expire in 2026 [para. 60][para. 61].
Overall, the article emphasizes that while weight loss metrics remain crucial, the broader health benefits, side effect management, and patient convenience are transforming how obesity treatments are developed and perceived [para. 86][para. 87].
- Eli Lilly and Company
礼来 - Eli Lilly and Company (LLY) developed Tirzepatide, a GLP-1/GIP dual receptor agonist used for weight loss. Approved by China's National Medical Products Administration in July, it competes with Novo Nordisk's Semaglutide in the weight loss market. The company is focused on scaling up production to meet high demand.
- Novo Nordisk
诺和诺德 - Novo Nordisk, a prominent player in the endocrinology field, has taken a leading position in the weight-loss drug market with its GLP-1 receptor agonists, liraglutide and semaglutide. Semaglutide, initially approved for diabetes treatment in 2017, later secured FDA approval for weight-loss in 2021. In China, semaglutide was approved for weight-loss in June 2024. The company is also exploring oral formulations and other dual-target drugs to maintain its competitive edge.
- Boehringer Ingelheim
勃林格殷格翰 - Boehringer Ingelheim, in collaboration with Zealand Pharma, is developing Survodutide, a GLP-1/GLP-2 dual receptor agonist. Currently in global phase III clinical trials, Survodutide has shown promising results, including 46-week average weight loss of 19% in phase II trials. It is also being explored for treating metabolic-associated steatohepatitis (MASH), with both FDA and China's NMPA granting breakthrough therapy designations.
- Pfizer Inc.
辉瑞 - Pfizer Inc. is actively involved in the GLP-1 drug development landscape. The company is progressing with its small molecule oral GLP-1 receptor agonist, Danuglipron. Phase IIb trial data revealed that Danuglipron achieved an average weight reduction of 6.9% to 11.7% over 32 weeks. Pfizer aims to enhance the formulation for once-daily dosing and advance it through further trials.
- Amgen Inc.
安进 - Amgen Inc. is developing AMG-133, a GIP/GLP-1 antibody peptide conjugate. Early clinical trials showed promising short-term weight loss, with phase I results indicating an 8.2% average weight reduction at 92 days from a single dose and a 14.5% reduction after monthly dosing for three months, sustained post-150 days. AMGEN plans to further test an extended dosing regimen before launching phase III trials.
- Zealand Pharma
Zealand Pharma - Zealand Pharma, in collaboration with Boehringer Ingelheim, is developing Survodutide, a GLP-1/GLP-2 receptor dual agonist. This drug is undergoing global multi-center Phase III clinical trials and has shown promising results in Phase II, with an average weight reduction of 19% over 46 weeks. Survodutide has also demonstrated significant improvement in treating metabolic associated steatohepatitis (MASH).
- Innovent Biologics
信达生物 - Innovent Biologics is involved in the Chinese market commercialization and clinical development of Mounjaro (玛仕度肽), a GLP-1/GCG dual receptor agonist developed by Eli Lilly. As of February 2024, Innovent has submitted an application for Mounjaro's weight loss indication to the National Medical Products Administration (NMPA), showcasing its active role in the GLP-1 medication landscape.
- AstraZeneca
阿斯利康 - AstraZeneca is advancing the development of a small molecule oral GLP-1 receptor agonist named AZD5004, which was acquired from Chugai. Having completed Phase I clinical trials with positive results in glucose control and weight loss, AstraZeneca is now proceeding with further clinical evaluations.
- 1890s:
- The development of weight loss drugs by humans can be traced back to this period.
- Before 2023:
- The only drug approved for weight loss indication and still available on the domestic market was Orlistat.
- April 2021:
- Semaglutide injections were approved for glycemic control in China.
- May 2022:
- Tirzepatide was first approved by the FDA for a glycemic control indication.
- April 2023:
- Eli Lilly initiated the SURMOUNT-5 clinical trial for comparing Tirzepatide and Semaglutide.
- By the end of 2023:
- Semaglutide ranked second in total global pharmaceutical sales.
- Before the end of 2023:
- The weight-loss product Wegovy had only been approved for two and a half years, yet its annual sales reached approximately $4.5 billion.
- November 2023:
- Tirzepatide received FDA approval for a weight-loss indication (overseas trade name: Zepbound).
- June 2024:
- Semaglutide injection was officially approved for weight loss indications in China.
- July 2024:
- The Chinese Society of Endocrinology released the 'Guidelines for Long-term Weight Management and Clinical Application of Drugs in Obese Patients'.
- August 2024:
- Qu Shen remarked positively about the clinical applicability of GLP-1 medications for obesity treatment.
- August 2024:
- Eli Lilly announced increased supply and price reduction for Zepbound.
- August 2024:
- A total of 338 clinical trials involving GLP-1 drugs for weight loss have been initiated or are ongoing globally according to ClinicalTrials.gov.
- August 18, 2024:
- Ji Linong gave an interview during the China Obesity Conference.
- June 26, 2024:
- Clinical trials for Amycretin tablets for weight loss indications approved in China.
- PODCAST
- MOST POPULAR





 Sign in with Google
Sign in with Google
 Sign in with Facebook
Sign in with Facebook
 Sign in with 财新
Sign in with 财新